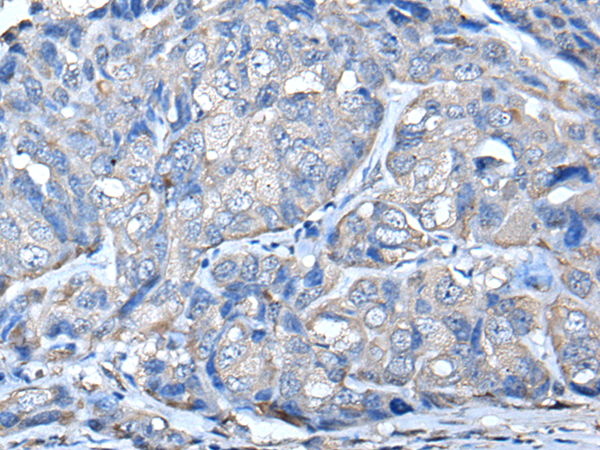
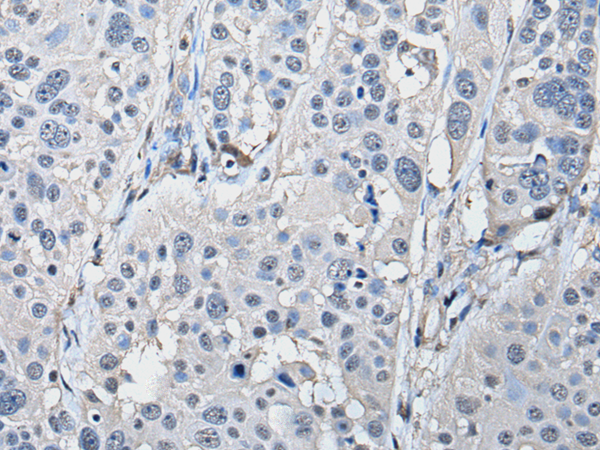
PRKAR2A Polyclonal Antibody
For reference only. Please follow the manual included in your kit for instructions.
Catalog Number
RD252196A
Product Name
PRKAR2A Polyclonal Antibody
Catalog Number
RD252196A
Clonality
Polyclonal
Purification Method
Antigen affinity purification
Isotype
IgG
Host
Rabbit
Background
cAMP is a signaling molecule important for a variety of cellular functions. cAMP exerts its effects by activating the cAMP-dependent protein kinase, which transduces the signal through phosphorylation of different target proteins. The inactive kinase holoenzyme is a tetramer composed of two regulatory and two catalytic subunits. cAMP causes the dissociation of the inactive holoenzyme into a dimer of regulatory subunits bound to four cAMP and two free monomeric catalytic subunits. Four different regulatory subunits and three catalytic subunits have been identified in humans. The protein encoded by this gene is one of the regulatory subunits. This subunit can be phosphorylated by the activated catalytic subunit. It may interact with various A-kinase anchoring proteins and determine the subcellular localization of cAMP-dependent protein kinase. This subunit has been shown to regulate protein transport from endosomes to the Golgi apparatus and further to the endoplasmic reticulum (ER).
Immunogen Information
Immunogen
Fusion protein of human PRKAR2A
Swissprot
P13861
Synonyms
cAMP dependent protein kinase regulatory subunit alpha 2cAMP dependent protein kinase regulatory subunit RII alphacAMP dependent protein kinase type II alpha regulatory chaincAMP dependent protein kinase type II alpha regulatory subunitcAMP-dependent
Calculated MW
46 kDa
Observed MW
Refer to figures
Gene Accession
BC002763
Applications
Reactivity
Human
Tested Applications
WB,IHC,ELISA
Conjugation
Unconjugated
Dilution
WB 1:500-1:2000, IHC 1:35-1:200, ELISA 1:5000-1:10000
Concentration
0.8 mg/mL
Storage Buffer
PBS with 0.05% NaN3 and 40% Glycerol, pH7.4
Storage Instructions
Store at -20°C. Avoid freeze / thaw cycles.