MMP-1 Polyclonal Antibody
For reference only. Please follow the manual included in your kit for instructions.
Catalog Number
RD90719A
Product Name
MMP-1 Polyclonal Antibody
Catalog Number
RD90719A
Clonality
Polyclonal
Purification Method
Affinity purification
Isotype
IgG
Host
Rabbit
Background
Matrix metalloproteinases are a family of zinc and calcium dependent endopeptidases with the combined ability to degrade all the components of the extracellular matrix. MMP-1 (interstitial collagenase), can degrade a broad range of substrates including types I, II, III, VII, VIII, and X collagens as well as casein, gelatin,alpha -1 antitrypsin, myelin basic protein, L-Selectin, pro-TNF, IL-1 beta, IGFBP-3, IGFBP-5, pro-MMP-2, and pro-MMP-9. A significant role of MMP-1 is the degradation of fibrillar collagens in extracellular matrix remodeling, characterized by the cleavage of the interstitial collagen triple helix into ?, ? fragments. However, as the list of substrates above illustrates, the role of MMP-1 is more diverse than originally envisaged, and may involve enzyme cascades, cytokine regulation, and cell surface molecule modulation. MMP-1 is expressed by fibroblasts, keratinocytes, endothelial cells, monocytes, and macrophages. Structurally, MMP-1 may be divided into several distinct domains; a pro-domain which is cleaved upon activation; a catalytic domain containing the zinc binding site; a short hinge region and a carboxyl terminal (hemopexin-like) domain.
Immunogen Information
Immunogen
Recombinant Human MMP-1 protein expressed by E.coli
Swissprot
P03956
Synonyms
MMP-1CLG
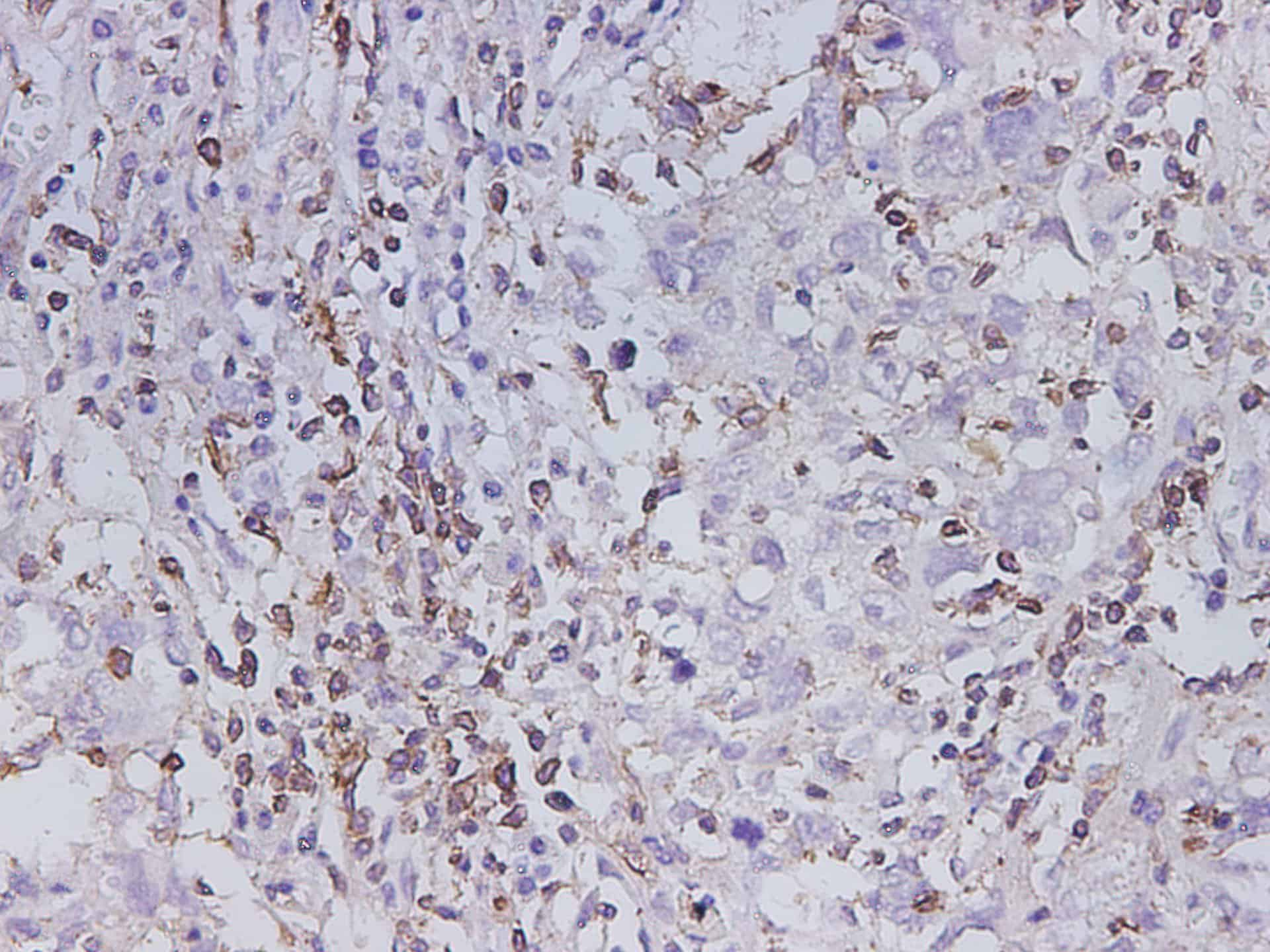
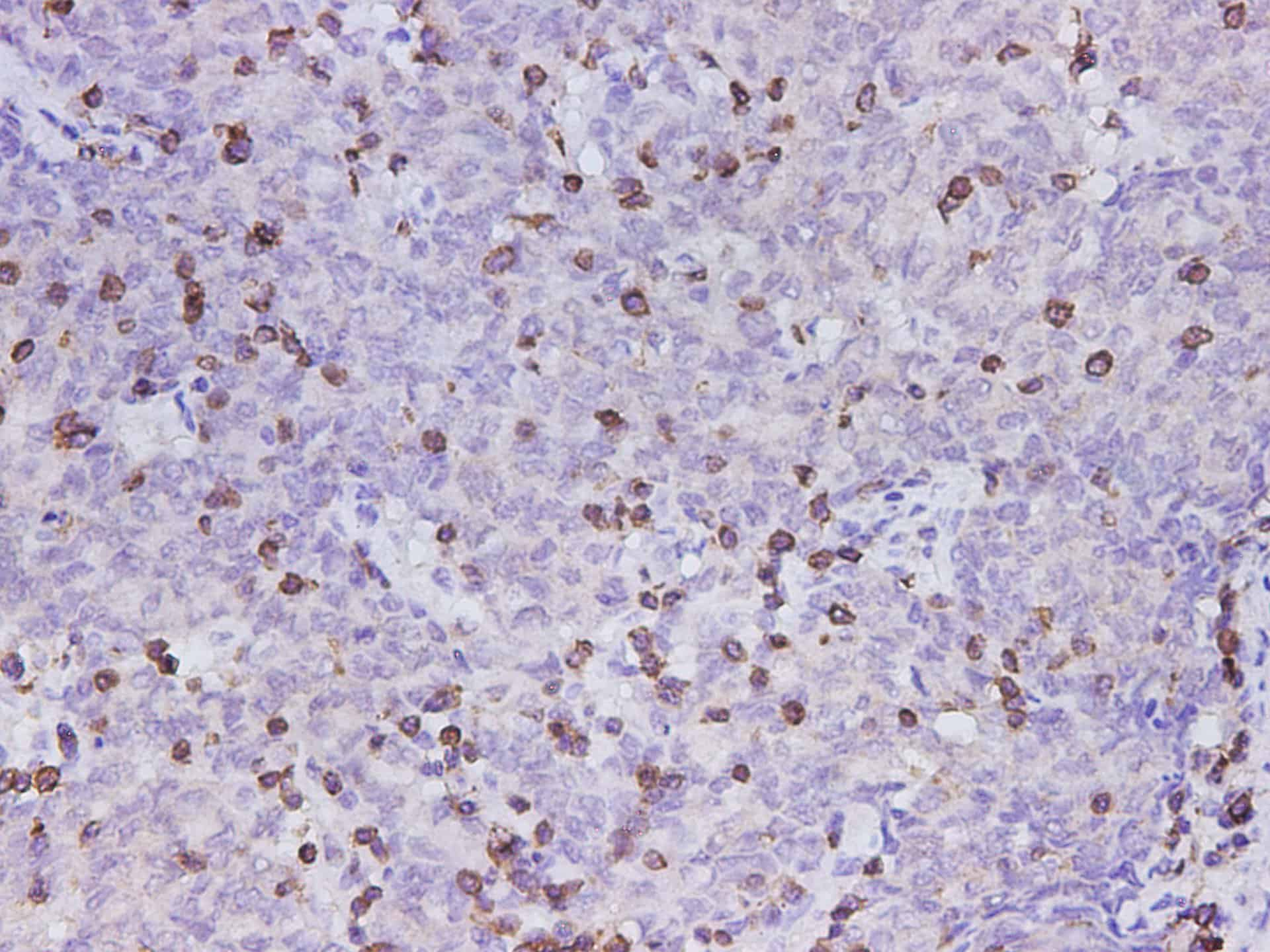
Applications
Reactivity
Human
Tested Applications
IHC
Conjugation
Unconjugated
Dilution
IHC 1:300-1:600
Concentration
1 mg/mL
Storage Buffer
PBS with 0.02% sodium azide, 50% glycerol, pH 7.4
Storage Instructions
Store at -20°C Valid for 12 months. Avoid freeze / thaw cycles.