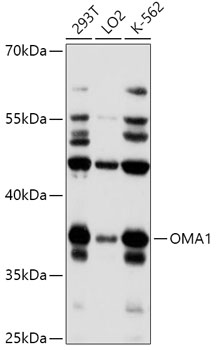
OMA1 Polyclonal Antibody
For reference only. Please follow the manual included in your kit for instructions.
Catalog Number
Product Name
OMA1 Polyclonal Antibody
Catalog Number
RD87806A
Clonality
Polyclonal
Purification Method
Affinity purification
Isotype
IgG
Host
Rabbit
Background
Metalloprotease that is part of the quality control system in the inner membrane of mitochondria. Activated in response to various mitochondrial stress, leading to the proteolytic cleavage of target proteins, such as OPA1, UQCC3 and DELE1. Following stress conditions that induce loss of mitochondrial membrane potential, mediates cleavage of OPA1 at S1 position, leading to OPA1 inactivation and negative regulation of mitochondrial fusion. Also acts as a regulator of apoptosis: upon BAK and BAX aggregation, mediates cleavage of OPA1, leading to the remodeling of mitochondrial cristae and allowing the release of cytochrome c from mitochondrial cristae. In depolarized mitochondria, may also act as a backup protease for PINK1 by mediating PINK1 cleavage and promoting its subsequent degradation by the proteasome. May also cleave UQCC3 in response to mitochondrial depolarization. Also acts as an activator of the integrated stress response (ISR: in response to mitochondrial stress, mediates cleavage of DELE1 to generate the processed form of DELE1 (S-DELE1, which translocates to the cytosol and activates EIF2AK1/HRI to trigger the ISR. Its role in mitochondrial quality control is essential for regulating lipid metabolism as well as to maintain body temperature and energy expenditure under cold-stress conditions (By similarity. Binds cardiolipin, possibly regulating its protein turnover (By similarity. Required for the stability of the respiratory supercomplexes (By similarity.
Immunogen Information
Immunogen
Recombinant fusion protein of human OMA1
Gene ID
115209
Swissprot
Q96E52
Synonyms
OMA12010001O09RikDAB1MPRP-1MPRP1YKR087CZMPOMA1peptidase
Calculated MW
88kDa
Observed MW
110kDa
Applications
Reactivity
Human,Mouse,Rat
Tested Applications
WB
Conjugation
Unconjugated
Dilution
WB 1:500-1:2000
Concentration
1mg/mL
Storage Buffer
PBS with 0.01% thiomersal,50% glycerol,pH7.3.
Storage Instructions
Store at -20°C Valid for 12 months. Avoid freeze / thaw cycles.