SIGLEC12 Polyclonal Antibody
For reference only. Please follow the manual included in your kit for instructions.
Catalog Number
Product Name
SIGLEC12 Polyclonal Antibody
Catalog Number
RD85157A
Purification Method
Affinity purification
Isotype
IgG
Host
Rabbit
Background
Sialic acid-binding immunoglobulin-like lectins (SIGLECs) are a family of cell surface proteins belonging to the immunoglobulin superfamily. They mediate protein-carbohydrate interactions by selectively binding to different sialic acid moieties present on glycolipids and glycoproteins. This gene encodes a member of the SIGLEC3-like subfamily of SIGLECs. Members of this subfamily are characterized by an extracellular V-set immunoglobulin-like domain followed by two C2-set immunoglobulin-like domains, and the cytoplasmic tyrosine-based motifs ITIM and SLAM-like. The encoded protein, upon tyrosine phosphorylation, has been shown to recruit the Src homology 2 domain-containing protein-tyrosine phosphatases SHP1 and SHP2. It has been suggested that the protein is involved in the negative regulation of macrophage signaling by functioning as an inhibitory receptor. This gene is located in a cluster with other SIGLEC3-like genes on 19q13.4. Alternative splicing results in multiple transcript variants.
Immunogen Information
Immunogen
Recombinant fusion protein of human SIGLEC12 (NP_443729.1).
Gene ID
89858
Swissprot
Q96PQ1
Synonyms
SIGLEC12S2VSIGLECL1SLGSiglec-XII
Calculated MW
51 kDa/64 kDa
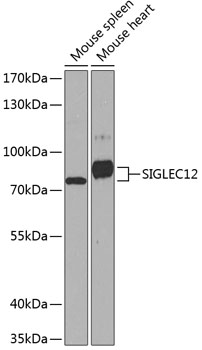
Observed MW
80 kDa
Applications
Reactivity
Mouse
Tested Applications
WB
Conjugation
Unconjugated
Dilution
WB 1:500-1:2000
Concentration
1 mg/mL
Storage Buffer
PBS with 0.02% sodium azide, 50% glycerol, pH7.3
Storage Instructions
Store at -20°C. Avoid freeze / thaw cycles.