STX5 Polyclonal Antibody
For reference only. Please follow the manual included in your kit for instructions.
Catalog Number
RD252597A
Product Name
STX5 Polyclonal Antibody
Catalog Number
RD252597A
Clonality
Polyclonal
Purification Method
Antigen affinity purification
Isotype
IgG
Host
Rabbit
Background
The membrane protein syntaxin 5 (STX5) is a key component of soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor (SNARE) complexes that regulate cellular protein transport, vesicle docking, and membrane fusion. Syntaxin 5 protein is found as a 42 kDa ("long") protein localized to the Golgi complex and endoplasmic reticulum, and a “short” 35 kDa isoform localized primarily to the Golgi. Formation of the syntaxin 5 SNARE complex, which also includes proteins Sec22B, Bet1, GOSR1, GOSR2, and Ykt6, allows for regulation of ER-to-Golgi transport, intra-Golgi transport, and endosome-to-Golgi retrograde transport. Research studies indicate that the syntaxin 5 SNARE complex also plays an essential role in autophagy following autophagosome formation. Intracellular protein transport mediated by the syntaxin 5 complex is required for transport and localized activity of lysosomal proteases. The experimental reduction or deletion of syntaxin 5 complex components results in non-functional lysosomes and accumulation of autophagosomes.
Immunogen Information
Immunogen
Fusion protein of human STX5
Swissprot
Q13190
Synonyms
OTTHUMP00000185028SED5STX5STX5STX5ASyntaxin 5Syntaxin 5ASyntaxin-5Syntaxin5Syntaxin5A
Calculated MW
40 kDa
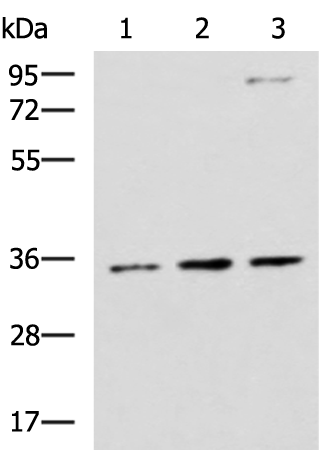
Observed MW
Refer to figures
Gene Accession
BC012137
Applications
Reactivity
Human, Mouse, Rat
Tested Applications
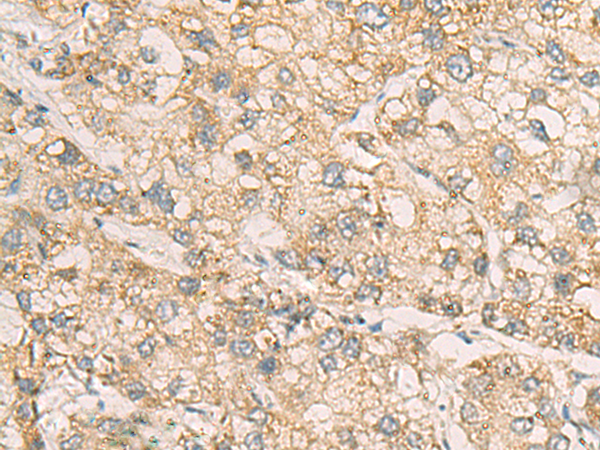
WB,IHC,ELISA
Conjugation
Unconjugated
Dilution
WB 1:500-1:2000, IHC 1:50-1:200, ELISA 1:5000-1:10000
Concentration
1.14 mg/mL
Storage Buffer
PBS with 0.05% NaN3 and 40% Glycerol, pH7.4
Storage Instructions
Store at -20°C. Avoid freeze / thaw cycles.