General 1,5-Anhydroglucitol (1,5-AG) ELISA Kit
For reference only. Please follow the manual included in your kit for instructions.
Catalog Number
The General 1,5-Anhydroglucitol (1,5-AG) ELISA Kit (RD-1,5-AG-Ge) is an enzyme-linked immunosorbent assay for quantitative detection of 1,5-AG in General samples.
This kit uses the Traditional ELISA format for colorimetric detection of natural 1,5-AG in samples including serum, plasma, tissue homogenates and other biological fluids.
The kit is easy to use and comes with a pre-coated microplate and all necessary reagents and buffers.
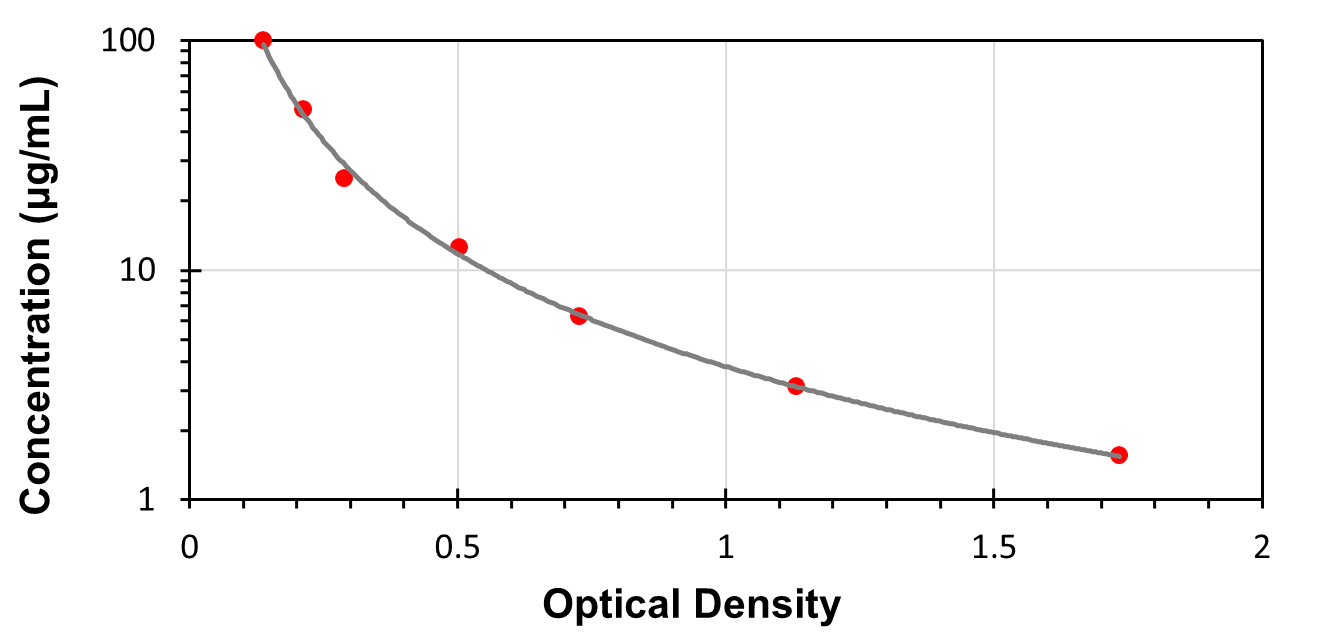
The standard curve generated in the experiment is used to calculate the concentration of 1,5-AG in samples.
For research use only.
Product Specifications
Product Name
General 1,5-Anhydroglucitol (1,5-AG) ELISA Kit
Catalog Number
RD-1,5-AG-Ge
Detection Range
1.56-100μg/mL
Sensitivity
0.52μg/mL
Species Reactivity
Species
General
Assay Length
4.5 hours
Experimental Method
Detection Method
Colorimetric
Sample Type
serum, plasma, tissue homogenates and other biological fluids
Shelf Life
12 months
Synonyms
LycoMark1-Deoxy-D-glucose1-Deoxy-D-glucopyranose1,5-Anhydro-D-glucitol1,5-AnhydrosorbitolAceritolPolygalytol
Product Details
Intended Use
The kit is a competitive inhibition enzyme immunoassay technique for the in vitro quantitative measurement of 1,5-AG in general serum, plasma, tissue homogenates and other biological fluids.
Test Principle
This assay employs the competitive inhibition enzyme immunoassay technique. A monoclonal antibody specific to 1,5-AG has been pre-coated onto a microplate. A competitive inhibition reaction is launched between biotin labeled 1,5-AG and unlabeled 1,5-AG (Standards or samples) with the pre-coated antibody specific to 1,5-AG. After incubation, the unbound conjugate is washed off. Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. The amount of bound HRP conjugate is reverse proportional to the concentration of 1,5-AG in the sample. After addition of the substrate solution, the intensity of color developed is reverse proportional to the concentration of 1,5-AG in the sample.
Application Data
Reagents and Materials Provided
| Reagents | Quantity | Reagents | Quantity |
|---|---|---|---|
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 2 |
| Standard | 2 | Diluent Buffer | 1 × 45 mL |
| Detection Reagent A | 1 × 70 μL | Detection Reagent B | 1 × 120 μL |
| TMB Substrate | 1 × 9 mL | Stop Solution | 1 × 6 mL |
| Wash Buffer (30× concentrate) | 1 × 20 mL | Instruction manual | 1 |
Materials Required but not Supplied
- Microplate reader with 450 ± 10 nm filter.
- Precision single or multi-channel pipettes and disposable tips.
- Eppendorf Tubes for diluting samples.
- Deionized or distilled water.
- Absorbent paper for blotting the microtiter plate.
- Container for Wash Solution.
Storage of the kits
- For unopened kits: All the reagents should be kept according to labels on the vials. The TMB Substrate, Wash Buffer (30× concentrate) and the Stop Solution should be stored at 4°C upon receipt while the others should be at -20°C.
- For opened kits: Once the kit is opened, the remaining reagents still need to be stored according to the above storage conditions. In addition, return the unused wells to the foil pouch containing the desiccant pack, and reseal along entire edge of zip-seal.
- For the expiration date of the kit, please refer to the label on the kit box. All components are stable until this date.
- It is highly recommended to use the remaining reagents within 1 month of opening.
Sample Collection and Storage
- Serum - Use a serum separator tube and allow samples to clot for 2 hours at room temperature or overnight at 4°C before centrifugation for 20 minutes at approximately 1000 × g. Assay freshly prepared serum immediately or store samples in aliquots at -20°C or -80°C for later use. Avoid repeated freeze/thaw cycles.
- Plasma - Collect plasma using EDTA or heparin as an anticoagulant. Centrifuge samples for 15 minutes at 1000 × g at 2-8°C within 30 minutes of collection. Remove plasma and assay immediately or store samples in aliquots at -20°C or -80°C for later use. Avoid repeated freeze/thaw cycles.
- Tissue homogenates - The preparation of tissue homogenates will vary depending upon tissue type. For this assay, tissues should be rinsed in ice-cold PBS (0.01 mol/L, pH 7.0-7.2) to remove excess blood thoroughly and weighed before homogenization. Mince the tissues to small pieces and homogenize them in 5-10 mL of PBS with a glass homogenizer on ice (Micro Tissue Grinders also work). The resulting suspension should be sonicated with an ultrasonic cell disrupter or subjected to 2 freeze/thaw cycles to further break the cell membranes. Then, centrifuge the homogenates for 5 minutes at 5000 × g. Remove the supernatant and assay immediately or aliquot and store at ≤-20°C.
- Other biological fluids - Centrifuge samples for 20 minutes at 1000 × g. Remove particulates and assay immediately or store samples in aliquots at -20°C or -80°C. Avoid repeated freeze/thaw cycles.
- Samples to be used within 5 days may be stored at 4°C, otherwise samples must be stored at -20°C (≤1 month) or -80°C (≤2 months) to avoid loss of bioactivity and contamination.
- Noticeable hemolysis will affect antibody-antigen reactions. Samples with any sign of hemolysis are not acceptable for this assay.
- When performing the assay, bring samples to room temperature.
Reagent Preparation
- Bring all kit components and samples to room temperature (18-25°C) before use.
- Standard - Reconstitute the Standard with 1.0 mL of Diluent Buffer, keep for 10 minutes at room temperature, shake gently (not to foam). The concentration of the standard in the stock solution is 100 μg/mL. Prepare 7 tubes containing 0.2 mL Diluent Buffer and use the diluted standard to produce a double dilution series according to the picture shown below. Mix each tube thoroughly before the next transfer. Prepare a dilution series with 7 points; for example: 100 μg/mL, 50 μg/mL, 25 μg/mL, 12.5 μg/mL, 6.25 μg/mL, 3.125 μg/mL, 1.562 μg/mL, and the last EP tube with Diluent Buffer is the blank at 0 μg/mL.
- Detection Reagent A and Detection Reagent B - Briefly spin or centrifuge the stock Detection A and Detection B before use. Dilute to the working concentration with Diluent Buffer respectively (1:100).
- Wash Solution - Dilute 20 mL of Wash Solution concentrate (30×) with 580 mL of deionized or distilled water to prepare 600 mL of Wash Solution (1×).
- TMB substrate - Aspirate the needed dosage of the solution with sterilized tips. Do not dump the residual solution back into the vial.
- Do not perform a serial dilution directly in the wells.
- Prepare standard within 15 minutes before assay. Do not dissolve the reagents at 37°C directly.
- Detection Reagent A and B are sticky solutions. Slowly pipette them to reduce the volume errors.
- Carefully reconstitute Standards or working Detection Reagent A and B according to the instruction, avoid foaming and mix gently until the crystals are completely dissolved. To minimize imprecision caused by pipetting, use small volumes and ensure that pipettors are calibrated. It is recommended to pipette more than 10 μL at a time to ensure accuracy.
- The reconstituted Standards, Detection Reagent A and Detection Reagent B can be used only once.
- If crystals have formed in the Wash Solution concentrate (30×), warm to room temperature and mix gently until the crystals are completely dissolved.
- Any contaminated water or container used during reagent preparation will influence the detection result.
Assay Procedure Summary
- Prepare all reagents, samples and standards.
- Add 50 µL standard or sample to each well. And then add 50 µL prepared Detection Reagent A immediately. Shake and mix. Incubate 1 hour at 37°C.
- Aspirate and wash 3 times.
- Add 100 μL prepared Detection Reagent B. Incubate 1 hour at 37°C.
- Aspirate and wash 5 times.
- Add 90 μL Substrate Solution. Incubate 15-25 minutes at 37°C.
- Add 50 μL Stop Solution. Read at 450 nm immediately.
Calculation of Results
This assay employs the competitive inhibition enzyme immunoassay technique, so there is an inverse correlation between 1,5-AG concentration in the sample and the assay signal intensity. Average the duplicate readings for each standard, control, and samples. Create a standard curve on log-log or semi-log graph paper, with the log of 1,5-AG concentration on the y-axis and absorbance on the x-axis. Draw the best fit straight line through the standard points, or it can be determined by regression analysis. Using plotting software, (for instance, curve expert 1.30), is also recommended. If samples have been diluted, the concentration read from the standard curve must be multiplied by the dilution factor.
Product Data
Sensitivity
- The minimum detectable dose of 1,5-AG is typically less than 0.52 μg/mL.
- The sensitivity of this assay, or Lower Limit of Detection (LLD) was defined as the lowest protein concentration that could be differentiated from zero. It was determined by adding two standard deviations to the mean optical density value of twenty zero standard replicates and calculating the corresponding concentration.
Specificity
- This assay has high sensitivity and excellent specificity for detection of 1,5-AG.
- No significant cross-reactivity or interference between 1,5-AG and analogues was observed.
- Limited by current skills and knowledge, it is impossible to perform all possible cross-reactivity detection tests between 1,5-AG and all analogues, therefore, cross reactivity may still exist.
Precision
- Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level 1,5-AG were tested 20 times on one plate, respectively.
- Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level 1,5-AG were tested on 3 different plates, 8 replicates in each plate.
- CV (%) = SD/mean × 100
- Intra-Assay: CV<10%
- Inter-Assay: CV<12%
Stability
- The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
- To minimize unnecessary influences on the performance, operation procedures and lab conditions, especially room temperature, air humidity, and incubator temperatures should be strictly regulated. It is also strongly suggested that the whole assay is performed by the same experimenter from the beginning to the end.