Total Aflatoxin (AF) ELISA Kit
For reference only. Please follow the manual included in your kit for instructions.
Product Specifications
Product Details
Intended Use
For the quantitative detection of Total Aflatoxin (AF) concentration in cereals, formula feed, edible oil, peanut, biscuit, beer, wine, soy sauce, and vinegar samples.
Assay Principle
This kit uses Competitive-ELISA as the method for the quantitative detection. It can detect Total Aflatoxin (AF) in samples, such as cereals, formula feed, edible oil, etc. This kit is composed of ELISA Microtiter plate, HRP conjugate, antibody working solution, standard and other supplementary reagents. The microtiter plate in this kit has been pre-coated with coupled antigen. During the reaction, AF in the samples or standard competes with coupled antigen on the solid phase supporter for sites of anti-AF antibody. Then Horseradish Peroxidase (HRP) conjugate is added to each microtiter plate well, and substrate reagent is added for color development. There is a negative correlation between the OD value of samples and the concentration of AF. The concentration of AF in the samples can be calculated by comparing the OD of the samples to the standard curve.
Application Data
Reagents and Materials Provided
| Reagent | Quantity |
|---|---|
| ELISA Microtiter plate | 96 wells |
| Standard Liquid | 1 mL each (ppb=ng/mL=ng/g) (0 ppb, 0.02 ppb, 0.04 ppb, 0.08 ppb, 0.16 ppb, 0.32 ppb) |
| HRP Conjugate | 5.5 mL |
| Antibody Working Solution | 5.5 mL |
| Substrate Reagent A | 6 mL |
| Substrate Reagent B | 6 mL |
| Stop Solution | 6 mL |
| 20×Concentrated Wash Buffer | 40 mL |
| Plate Sealer | 3 |
| Sealed Bag | 1 |
| Manual | 1 |
All reagent bottle caps should be tightly closed to prevent evaporation and microbial contamination.
Materials Required but not Supplied
- Equipment: Microplate reader, Printer, Homogenizer, Nitrogen Evaporators, Water bath, Vortex mixer, Centrifuge, Graduated pipette, Balance (sensibility 0.01 g).
- Micropipettes: single-channel 20-200 µL, 100-1000 µL, and multi-channel 300 µL.
- Reagents: Methanol, N-hexane, Trichloromethane or Dichloromethane.
Storage
- Store the kit at 2-8℃. Do not freeze any kit components.
- Return any unused microwells to their original foil bag and reseal them together with the desiccant provided. Continue to store at 2-8℃.
- Shelf life is approximately 12 months from date of manufacture. Check packaging for expiry date.
Sample Preparation
- Cereal:
- Homogenize the representative sample with a homogenizer and mix fully.
- Weigh 2±0.05 g of homogenate sample into the 50 mL centrifuge tube, add 5 mL of 70% Methanol (Solution 1), vortex for 5 min, centrifuge at 4000 r/min for 10 min at room temperature.
- Take 0.5 mL of supernatant to another centrifuge tube, add 0.5 mL of deionized water, and mix fully.
- Take 50 μL for analysis.
- Formula feed:
- Homogenize the representative sample with a homogenizer and mix fully.
- Weigh 2±0.05 g of homogenate sample into the 50 mL centrifuge tube, add 10 mL of 70% Methanol (Solution 1), vortex for 5 min, centrifuge at 4000 r/min for 10 min at room temperature.
- Take 0.5 mL of supernatant to another centrifuge tube, add 0.5 mL of deionized water, and mix fully.
- Take 50 μL for analysis.
- (If aflatoxin content is higher in the sample, take the mixed liquid from step 2, diluted with 35% Methanol, the sample dilution multiple is the actual dilution multiple at the moment. For example: take the mixed liquid from step 2, diluted 10 times with 35% Methanol, the actual dilution multiple is 10×10=100, detection limit: 2 ppb)
- Edible oil, peanut, high fat formula feed:
- Homogenize the representative sample with a homogenizer and mix fully.
- Weigh 2±0.05 g of homogenate sample into the 50 mL centrifuge tube, add 8 mL of N-hexane and 10 mL of 70% Methanol (Solution 1), vortex for 5 min, centrifuge at 4000 r/min for 10min at room temperature.
- Discard the upper liquid, and take 0.5 mL of lower liquid to another centrifuge tube, add 0.5 mL of deionized water, mix fully.
- Take 50 μL for analysis.
- Biscuit:
- Homogenize the representative sample with a homogenizer and mix fully.
- Weigh 2±0.05 g of homogenate sample into the 50 mL centrifuge tube, add 10 mL of 70% Methanol (Solution 1), vortex for 5 min, centrifuge at 4000 r/min for 10 min at room temperature.
- Take 2 mL of supernatant to another centrifuge tube, add 4 mL of Trichloromethane or
- Dichloromethane, vortex for 5 min, centrifuge at 4000 r/min for 10 min at room temperature.
- Take the upper liquid to another centrifuge tube, keep the lower liquid for use (lower liquid A). Add 4 mL of Trichloromethane or Dichloromethane to the upper liquid, vortex sufficiently for 5 min, and centrifuge at 4000 r/min for 10 min at room temperature. Discard the upper liquid and keep the lower liquid (lower liquid B).
- Mix lower liquid A and lower liquid B thoroughly.
- Take 2 mL of mixed lower liquid and dry with nitrogen evaporators or water bath at 50-60℃(Please do it in a ventilated environment.).
- Add 0.5 mL of 70% Methanol (Solution 1) to dissolve thoroughly, add 0.5 mL of deionized water, and mix fully.
- Take 50 μL for analysis.
- Beer:
- Stir beer thoroughly to remove CO2, take 2 mL of beer sample and add 1 mL of deionized water, then add 7 mL of Methanol, vortex for 5 min.
- Take 0.5 mL of mixed sample liquid and add 0.5 mL of deionized water to another centrifuge tube, mix fully.
- Take 50 μL for analysis.
- Wine, soy sauce, vinegar:
- Take 2 mL of sample and add 2 mL of deionized water, then add 10 mL of Trichloromethane or Dichloromethane, vortex for 5 min, centrifuge at 4000 r/min for 10 min at room temperature.
- Remove all upper liquid. Take 1 mL of lower liquid to another centrifuge tube and dry with nitrogen evaporators or water bath at 50-60℃.(Please do it in a ventilated environment.)
- Add 0.5 mL of 70% Methanol (Solution 1) to dissolve thoroughly, add 0.5 mL of deionized water, and mix fully.
- Take 50 μL for analysis.
Reagent Preparation
- Bring all reagents and samples to room temperature before use.
- Turn on the microplate reader 30 min in advance to preheat the instrument and set the testing parameters.
- All experimental apparatus should be clean, and disposable pipette should be used and changed frequently to avoid cross-contamination during the experiment.
Assay Procedure
Bring all reagents and samples to room temperature (25℃) before use. All reagents should be mixed thoroughly by gently swirling before pipetting. Avoid foaming. Any unused Microtiter plate wells should be resealed as soon as possible and stored at 2-8℃.
- Number: number the samples and standards in order (multiple wells), and keep a record of the plate layout. Standards and Samples must be tested in duplicate.
- Add Sample: add 50 μL of Standard or Sample to each well, then add 50 uL HRP Conjugate, then 50 μL of Antibody Working Solution to each well, and cover the plate with plate sealer. Oscillate gently for 5 s to mix thoroughly, then incubate at 25℃ for 30 min in the dark.
- Wash: Carefully remove the plate sealer, then remove the liquid from each well. Immediately add 300 μL of Wash Buffer (Solution 2) to each well and wash. Repeat wash procedure 5 times, 30 s intervals each time. Invert the plate and pat it against a thick sheet of absorbent paper (If there are bubbles in the wells, clean pipette tips can be used to pop them).
- Color Development: add 50 μL of Substrate Reagent A to each well, and then add 50 μL of Substrate Reagent B. Gently oscillate for 5 s to mix thoroughly. Incubate at 25℃ for 15 min in the dark (The reaction time can be extended according to the amount of color change).
- Stop Reaction: add 50 μL of Stop Solution to each well. Gently oscillate for 10 s to mix thoroughly.
- OD Measurement: determine the optical density (OD value) of each well at 450 nm (reference wavelength 630 nm) with a microplate reader. This step should be performed no later than 10 min after the reaction has been stopped.
Calculation of Results
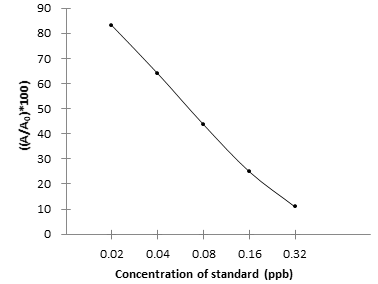
Absorbance (%) = A/A₀×100%
- A: Average absorbance of standard or sample
- A₀: Average absorbance of 0 ppb Standard
- Drawing and calculation of standard curve
- Create a standard curve by plotting the absorbance percentage of each standard on the y-axis against the log concentration on the x-axis to draw a semi-logarithmic plot. Add the average absorbance value of each sample to the standard curve to calculate the corresponding concentration. If samples have been diluted, the concentration calculated from the standard curve must be multiplied by the dilution factor.
- We recommend using professional software for fast and accurate analysis of large numbers of samples.